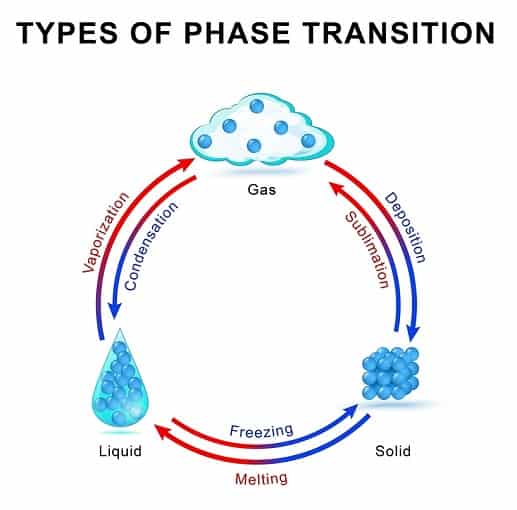
Sublimation is the process of conversion of a solid directly into a gas without forming a liquid.
In general, when a substance is heated, it melts to a liquid at a specific melting point.
Further, when this molten liquid is heated, it converts into a gas at boiling point temperature.
The intermediary step of liquid formation is absent in sublimation.
So the process of conversion of different states of matter is solid converts to liquid and this liquid boils out to give gas.
Solid →liquid→Gas (normal transition of matter).
Solid→ Gas (Sublimation phenomenon)
So the process is devoid of liquid state during transition.
What is sublimation process?
- In general, matter has particles bound together by inter-molecular and intra-molecular forces.
- These forces help keep the matter bulky, hard and stable.
- When heat energy is given to the matter, the forces of attraction are overcome due to vibrations of atoms in the molecules leading to a loss of stability and hardness.
- The molecules tend to arrange themselves in a loose fashion such that they start flowing like a liquid without any shape.
- On providing further energy, these molecules begin to vibrate with greater intensity than before and tend to move apart from each other.
- It exists as gas, which has loosely bound molecules.
- These molecules are free to move and try to occupy the open space as much as possible.
- But volatile substances tend to directly convert from solid to gas at room temperature.
- This phenomenon of conversion directly from solids to gases is called sublimation.
Examples of sublimation include
1. Naphthalene
When used as an insecticide and put between clothes, we can notice that it loses weight gradually. This loss of weight is due to sublimation. Naphthalene ball converts slowly to gas on standing. This gas is toxic to insects and hence they stay away from clothes.
2. Water
- Even water shows this behavior at triple point.
- Ice converts to water and this water converts to water vapor (gas) by normal heating.
- But at a triple point temperature of 273.1600 K and pressure of 611.657 pascals, ice converts directly into gas.
3. Iodine
- Iodine is a good example of sublimation material. The crystals of it sublime slowly at normal room temperature.
Uses of sublimation
Drying
- Sublimation helps to dry substance within getting moist. This process of sublimation is also beneficial in medicine.
- It is used in making powders from heat-sensitive materials etc.
Purification
- If is a substance is in contaminated form; it can be separated into pure form by sublimation technique.
- Since the sublimation temperature of substance is different than the contaminant, by sublimation, the substance is separated, leaving the contaminant behind.
Aroma
- Sublimation is the principle involved in air freshener solids used in rooms and toilets.
- The room fresheners are solids packed in a box. They spread the odor to the entire room by getting evaporated.