Oxygen is a vital gas responsible for life on earth. It generates energy and supports the life of all living organisms. With its elemental properties, it finds many applications as below.
10 Important Benefits of Oxygen include
- Energy generation in the body.
- Health care
- Poison management.
- Gas Welding
- Combustion of fuels in automobiles.
- In sterilization.
- For sanitation.
- Chemical Analysis (quality control)
- Involved in chemical reactions.
- In industry
Supplies Energy to the body
- We all live because of our body cell’s ability to produce energy from food in the presence of oxygen.
- This energy is generated in the form of ATP (adenosine triphosphate).
- We breathe oxygen from the air, which is carried by the hemoglobin in blood from the lungs. It is distributed to all the cells and tissues of the body.
- There, the oxygen is taken up by the mitochondria in the cells and is used to break down the glucose molecule from food to produce energy (ATP).
The reaction is shown in the picture below.
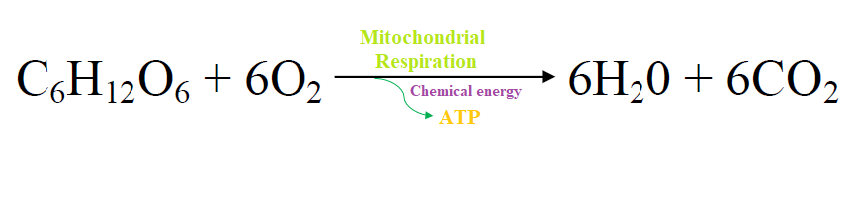
- The above reaction produces water and carbon dioxide as waste products which are expelled.
Thus, it is a life-generating gas and therefore carried into space and also during deep-sea diving.
- Animals living in water also take up oxygen with the help of gills. Hence, the air-water combination is critical even in aquariums, as the fishes need to take in oxygen.
- Even the plants also take up oxygen for the production of the required energy. However, they also produce oxygen by photosynthesis.
In Health Care
- Oxygen gas is useful in the treatment of respiratory insufficiency diseases.
- For diseases like COPD, pneumonia, COVID and heart failure, oxygen therapy is given where oxygen is combined with carbon dioxide gas for inhalation.
- Due to insufficient breathing, there is a strain on the heart to manage oxygen demand through blood circulation.
- This leads to heart problems and even death.
- When oxygen therapy is given, the amount of oxygen gas given is higher than that present in the normal air. Hence, it reaches the body tissues even if the lungs malfunction.
For this medical use, liquid oxygen is used as a means to store large volumes of oxygen. One liter of liquid oxygen yield 860 liters of gaseous oxygen.
- Even in the case of poisoning, when the respiratory center is compromised, oxygen therapy may be given to stabilize the patient.
- Thus medical oxygen is an essential aid in critical conditions to save lives and buy time for further procedures.
In Cyanide Poisoning management
- In the case of poisoning by cyanide and carbon monoxide, oxygen can help save a life.
- Cyanide binds to mitochondrial complexes and blocks the energy supply leading to a quick death.
- Oxygen, when given in high doses, competes with cyanide to bind to mitochondria and delays the poisoning, thereby buying time to detoxify the body.
- Thus it paves the way for the detoxification of cyanide using the antidote sodium thiosulfate.
- Similarly, when there is carbon monoxide poising, patients are subjected to hyperbaric oxygen therapy, which replaces CO present in the blood and tissues fast.
In Gas welding

- Welding is a process of binding metal surfaces at high temperatures.
- Oxygen is used in gas welding instead of regular air.
- The use of oxygen is said to increase the flame temperature, thereby helping in the localized melting of metal at the point of exposure.
For example, compare the heat energy when fuel propane is burnt with air and oxygen in the table below.
| Fuel | Ignition gas | Heat generated (Temperature) |
| Propane | Air | 1,980 °C |
| Propane | Oxygen | 2,253 °C |
- So, when welding fuel is burnt by oxygen, it generates higher energy.
- High temperature leads to faster melting of metals resulting in efficient welding.
For the combustion of fuels in automobiles
- Oxygen is required for the burning of substances or fuel.
- Most automobiles we see in daily life run on petroleum products.
- Petrol is needed to generate mechanical energy inside the closed combustion engines.
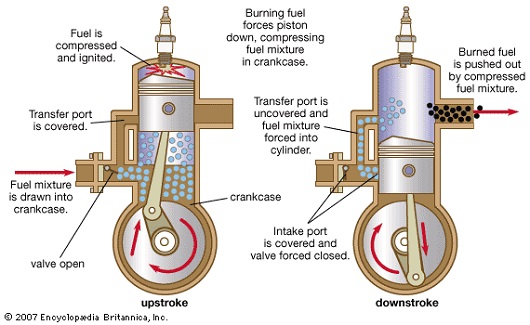
- As seen in the above picture, oxygen reacts with fuel and burns to generate heat energy.
- This heat energy converts to mechanical energy as it pushes the pistons in the cylinder.
- Thus, oxygen is required to run automobiles. Without oxygen, it would be difficult to burn fuels.
- So when the air is sent into the combustion engine, the oxygen is for the burning of fuel. There are separate air filters in the bike or car to pass on air free of dust into the engine.
For sterilization
- Oxygen is a strong sterilizing agent due to its oxidizing property.
- It can destroy microbes in a given environment, whether water or land.
- It is used in the form of hydrogen peroxide H2O2 as a disinfectant to kill microbes on surgical equipment.
- Also, there is a method called oxygen plasma treatment whereby an oxygen molecule is broken down to release a single oxygen atom or its ions, which are deadly toxic.
- They are used for the sterilization of substances at room temperature.
For sanitation
- Oxygen gas is also used for the decay of waste and the sanitation of our environment.
- Sanitation is measured in terms of BOD and COD.
- BOD means biological oxygen demand, and COD means chemical oxygen demand.
- When we take them in detail, BOD is the amount of dissolved oxygen required by bacteria or other microbes to destroy the waste.
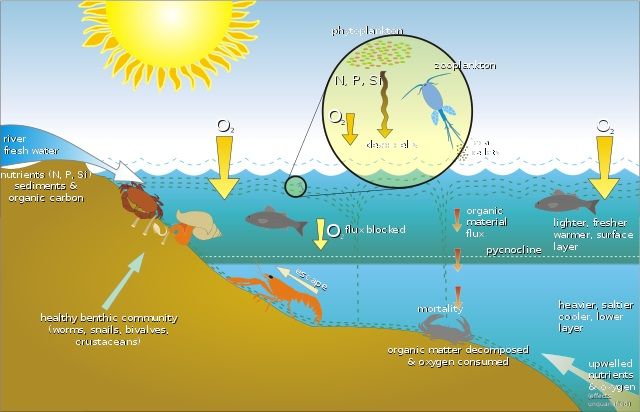
- Sometimes this oxygen consumption can be severe, leading to oxygen depletion and the death of aquatic animals.
- COD means the amount of chemicals needed to destroy a certain amount of waste.
- In both, the processes of oxygen are involved in destroying the waste. Read more details on biological oxygen demand.
- Even we can notice metals like iron destroyed by oxidation when exposed to air for a long time.
- This is called rust, but actually, it is the oxidation of the metal due to oxygen in the air.
- Hence oxygen is a potent agent that can destroy substances.
- Though, in our terms, this destruction by rust formation is disadvantageous, it is the law of nature to derange substances.
- So oxygen supports this natural law by destroying them.
For chemical reactions
- Here an important chemical properties of oxygen i.e, its high electronegativity is ulitized. This ability to take electrons helps in many oxidation-reduction reaction-based chemical reactions.
- In this reaction, there is an addition or removal of oxygen.
- Besides oxygen, hydrogen, and electrons are also involved in the process.
- It reacts with many organic and inorganic substances to produce new compounds.
- These chemical reactions are used for synthesis, analysis, titration and also for degradation purposes.
For analysis of substances
- Sample analysis is a key aspect of quality control in the industry.
- Some of the analysis methods like flame photometry and atomic absorption spectrometry use an oxygen fuel-based flame to help determine the elements in the sample.
- Here oxygen helps generate a flame of high temperature so as to convert sample drops into molecules and atoms to facilitate analysis.
In industry
Oxygen is used for smelting iron ore to produce steel and also as rocket fuel to propel the craft.