Spectroscopy is a branch of physics that deals with the interaction of light with materials.
In other words, it is an analytical method for qualitative and quantitative estimation by use of light.
Light is as we all know is electromagnetic radiation which has wave and frequency as measurable characters.
You can know more details here on the principle of spectroscopy.
Spectroscopy finds a widespread application in daily life. It is used in analytical chemistry, phytochemistry (plant chemistry), biological analysis, health care, and medicine.
Further is became a part of other means of estimation and analysis like chromatography, elemental analysis, and also identification tools.
This is mainly of 3 types which are again classified into subtypes.
The different types of spectroscopy include
1) Atomic absorbtion spectroscopy
- Here, the energy changes occur at the atomic levels.
- The measurement is done to study the specific atoms and their quantity.
- The samples under test are sprayed into a flame to obtain neutral atoms.
- These atoms absorb light of specific wavelength generated by hollow cathode lamp.
- By this atomic absorption spectroscopy metals like Sodium, calcium, magnesium-related formulations are measured.

Atomic emission spectroscopy (flame photometry)
This is similar to the above atomic absorption spectroscopy but here instead of absorption, the emission from atoms is measured.
- The sample solution is sprayed onto a flame as fine droplets.
- As the solvent evaporates, the residue converts into neutral atoms.
- The thermal energy of flame leads to excitation of the neutral atoms.
- The exited state is unstable and hence, the atoms return to ground state emitting radiation of specific wavelength.
- The emitted radiation of specific wavelength is characteristic of an element and helps to analyze the quantity of sample based on the intensity of radiation.
- These are referred to as flame emission spectroscopy.
- The above two techniques finds limited applications as the characters of most substances are dependent on the molecular nature and not just atoms in it.
Also, the methods are expensive, time-taking and even quite tedious to perform.
Methods based on Molecular spectroscopy
These method evaluates the energy changes that occur at the molecular level.
The characters like molecular absorption, emission, and vibration are studied.
Examples include
- UV-spectroscopy (includes Colorimetry)
- Infrared,
- FTIR,
- Fluorimetry.
- This method of spectroscopy is widely preferred due to many applications.
- The process is quick, easy, and results are accurate.
Further, these methods of spectroscopy can be integrated into other analytical techniques like chromatography.
B) Based on the property of either absorption or emission.
Here the principle of abortion or emission of electromagnetic radiation is taken into consideration.
1) Absorption spectroscopy: As the name suggests, here there is the absorption of light by the sample. The extent of absorption and the wavelength of the absorbed light is considered. The wavelength of light absorbed tells the nature of the compound while the intensity of absorbed light tells the concentration.
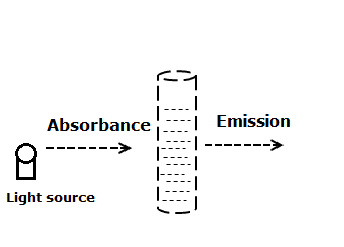
The examples of the spectroscopic methods coming under this method are colorimetry, UV-spectroscopy, infrared spectroscopy, NMR spectroscopy, atomic absorption spectroscopy.
2) Emission spectroscopy: Contrary to the above method, here the emitted light is measured. once the light impinges on the sample, some of it is absorbed. This absorption of light leads to the transition of electrons from the ground state to the excited state. These excited electrons return back to the ground state by the release of electromagnetic radiation (light) of a specific wavelength. The intensity gives the concentration while the wavelength tells the nature of the compound.
Examples include fluorescence spectroscopy, flame photometry.
C) Based on the level of study i.e. electronic or magnetic levels.
Here the study is done based on the electronic or magnetic properties of the compound. Light is electromagnetic radiation. That is it has both electronic and magnetic properties.
Electronic spectroscopy: So when a compound is estimated without the magnetic field we call it electronic spectroscopy. In this method, the substance under test is exposed to light without the influence of the magnetic field.
Examples of this method are again colorimetry, UV visible spectroscopy, IR, fluorimetry, etc.
Magnetic spectroscopy: Here the substance is exposed to electromagnetic radiation in presence of an external magnetic field.
Examples include Nuclear magnetic resonance spectroscopy (NMR), Electron spin resonance spectroscopy (ESR).
informative and well data structure.
easily understandable