There are three states of matter in nature like the solid, liquid, and gas. The li
Both liquids and gases are materials that tend to flow at room temperature.
This physical property to flow is due to the loose arrangement of molecules within the matter.
And this loose arrangement is again due to the weak intermolecular forces of attraction compared to solids.
Hence unlike solids, they cannot retain a shape on their own. Instead, they occupy the shape of the vessel they are poured into.
Interestingly, the gases have further weaker forces of attraction between molecules, and hence, they vaporize on exposure to air.
Types of liquids
In nature, there 6 different kinds of liquids available in 6 different types, as below.
- Solvents
- Solutions
- Emulsions
- Suspensions
- Aerated liquids.
- Liquid gases
Solvents
These are the plain liquids on the list as they are made up of the same set of molecules.
One can say they are pure compounds as they are made of only one set of molecules absolutely.

For example, water is a liquid and solvent made up of H2O molecules, and ethanol is made up of C2H5OH molecules.
In fact, they are the most common liquids we use in everyday life.
These liquids can be obtained in large quantities from nature.
Ex: Water, ethanol, plant oils, mineral oils, organic solvents like benzene, carbon tetrachloride, acetone, etc.
- Some of these solvents are used to prepare other types of liquids like solutions, suspensions, emulsions, and aerated liquids.
- Without these solvents, other liquids cannot be made.
Based on their chemistry, nature, polarity, pH, etc., these solvents are of different types.
Solutions
- These are liquids that have two or more substances dissolved.
- For example, when we mix sugar or salt into water, we get a solution.
Seawater is an example of a natural solution.
- When the substance is mixed with water (solvent), the substance molecules get separated from each other and disperse into the solvent.
- These dispersed molecules of the solid substance arrange themselves within the intermolecular spaces of solvent molecules.
- Thus you can notice that when you add table salt to a glass full of water, it does not overflow immediately. But overflows only after a certain amount of salt is further added.
- This indicates that some amount of salt occupies the intermolecular space between water molecules.
- With the further addition of salt, the water overflows due to a lack of extra space within the water.
Emulsions
- These are unique types of liquids wherein two insoluble (immiscible) liquids are virtually mixed up.
- One of the liquids is distributed as fine globules into the other.
In nature, such an example of emulsions is the “butter.”
- Even when our hand has oil, washing with plain water does not remove the soap entirely.
- But, when we use soap and water, it is removed.
- This is because soap allows the water to form an emulsion with oil on the hand and slips away from the skin’s surface.
There are different types of emulsions like the Water in Oil (W/O) type or Oil in Water (O/W) type.
- In the water in the Oil type, the water is distributed as fine globules into Oil and vice-versa.
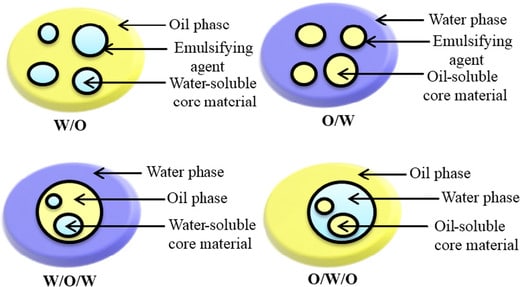
- These basic emulsions are further made into the water in oil in water (w/o/w) type and oil in water in oil type (o/w/w).
- These are called complex emulsions and have less stability.
- Many drugs are formulated in emulsion type as they are insoluble. Hence emulsions serve as drug delivery systems.
Suspensions

- These are the liquids wherein solid particles are suspended in a solvent.
- Unlike the solutions where the solids are dissolved in a solvent, here, the solid particles remain suspended without getting dissolved.
- These types of liquids are rarely found in nature around.
Muddy water and water mixed with fine sand (silt) are the only available natural suspensions.
- But the suspensions are prepared and widely used by humans in medical forms.
- Drug preparations like antacids are in the suspension form.
- But the suspended particles tend to settle down at the bottom of the liquid if kept still for a long time.
- Hence the label of the medical formulation carries the instruction “Shake well before Use.”
- Nowadays, nano-particle-based suspensions are also made in which the suspended particles are even smaller and do not settle down at the bottom.
Aerated solutions

- These are the solutions where gas is mixed with solvent or solutions.
- These are also less common in nature but are made as beverages by humans.
Coke and soda, which see in daily life, are examples.
- Here gas is entrapped within the liquid spaces. This gas gets expelled at a warm temperature.
- They are similar to solutions with the only difference being that instead of solid, gas is mixed with liquid.
- But unlike solutions, they have few differences in behavior.
- When shaken, the gas molecules get together to form bubbles.
- These bubbles put pressure on the solvent to expel it forcefully from the container.
- Hence we can notice foam when shaken.
- Also, to make a solution, a good amount of work is involved in dispersing solids particles into the liquid.
- But, for these, just passing gas into a liquid is sufficient.
Liquefied gases
- These are pure gases in liquid form.
- They are formed when the gas is cooled to a very low temperature.
For example, nitrogen converts to liquid nitrogen at −195.8 degrees temperature.
- In practice, we notice liquids, when heated to the boiling point, convert into a gaseous vapor.
- Similarly, when gases are subjected to extremely low temperatures, they convert into gaseous liquids.
Example of such liquids is liquid nitrogen, liquid oxygen, liquid hydrogen, etc.
These are used for special purposes and have to be handled with care as they can be destructive.
very well explained. Thank you
Wow what a good explanation about liquids. thank you
Nice!
Very well explained
Thank you
Excellent! Thank you.