Emulsions are bi-phasic liquid solutions.
They are used for drug delivery in medicine, cleaning oil and greasy surfaces, storing substances, etc.
The emulsified forms of medicine are administered by different routes like oral, parenteral, and even a few topical preparations like creams.
The emulsion has two liquids and of them, one liquid phase is evenly spread in the other liquid phase.
The one which is in a larger proportion is called the external phase. The one which is in lower ratios is called the internal phase.
And the internal phase and external phase liquids are insoluble in each other.
But, still, they are made to disperse with each other by an alteration of surface properties of the liquids involved.
The internal phase liquid is converted to tiny droplets with a suitable coating. This coating helps the internal phase to admix with the external phase.
If the bonding layer is destroyed, the emulsions break up leaving two immiscible liquids.
Types of emulsions
- Emulsions are basically of two types.
- But by careful and controlled mixing, they can be made into 2 additional types.
- So in total, there are 4 types of emulsions
Simple emulsions
The first type is called simple emulsions. They are as
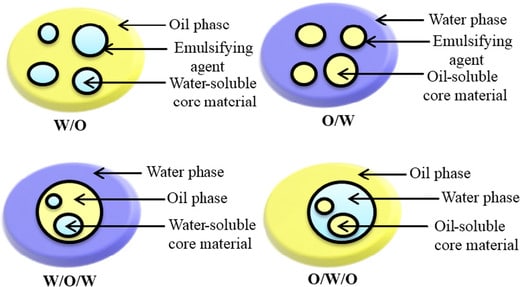
- W/O types emulsions
- O/W type of emulsions.
Complex emulsions
The other type is called complex emulsions. They are again of two types as
- W/O/W type emulsions
- O/W/O type of emulsions.
W/O type of emulsions
- These emulsions are the type wherein water is an internal phase and oil is an external phase.
- They can be identified by adding oil-soluble dyes. If the dye is oil soluble, the dye colors the whole solution.
O/W type of emulsion
- These emulsions have oil in an internal phase and water in the external phase.
- This emulsion can be identified by its electrical conducting nature.
- Since water is a good conductor, when an electrode is placed, it shows conductivity, indicating it to be an external phase.
W/O/W type of emulsions
Here water comprises two layers while oil is a center layer.
O/W/O type of emulsions
- Here water is the centermost layer while oil occupies the innermost and also the outermost layer.
How do emulsifiers work
- Emulsifiers are substances that can be soluble in both water and oil.
- When this substance is added to the solution and mixed, it forms layers around the internal phase particles or globules.
- Since it has an affinity for the other phase, too, it evenly disperses the globules throughout the external phase.
Emulsion Examples
Medicines like vitamins, steroids, and a few IV infusions.