Nucleic acids are molecules of high molecular weight formed by thousands of nucleotides.
Nucleic acids are of two types- deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). The primary function of the nucleic acids is to store and transmit genetic information from one generation to another.
Nucleic acids can be digested by lysosomic enzymes.
Nucleic Acid Chemistry and Structure
Structure
Nucleic acids are linear polymers. They are composed of the polymerization of monomeric units. These monomeric units in the case of nucleic acids are nucleotides. Thus nucleic acids could also be called polynucleotides. The nucleotides are joined successively to each other by 3’ and 5’ phosphate bridges.
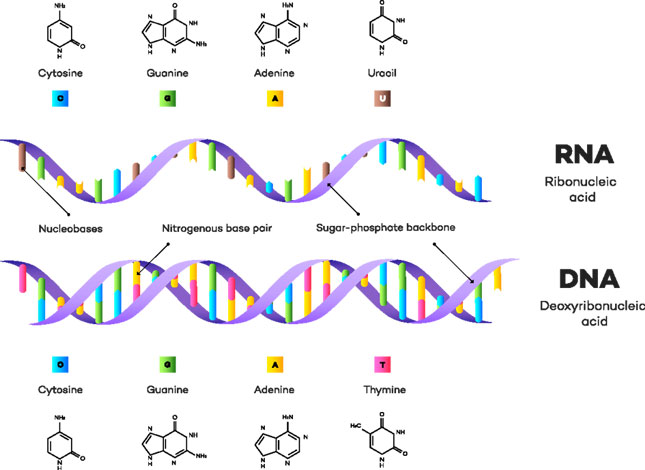
In each nucleotide, there is a nitrogenous base, a pentose sugar and a phosphate.
Nucleotides also participate in the formation of B-complex vitamins. Nucleotides are components of some coenzymes of B-complex vitamins. They are structural components of FAD, NAD+. They participate in respiration processes and other reactions related to energy production and subsequent usage in cells.
They also help in the regulation of metabolic reactions.
The term nucleotide refers to the combination of a nucleoside and a phosphate. A nucleoside is a combination of a sugar and a nitrogenous base (called nucleobase).
Nitrogenous bases-The nitrogenous bases making up the structure of nucleotides (which make up nucleic acids) are aromatic in nature. They also possess a heterocyclic ring structure. The bases present are of two types-purines and pyrimidines.
Purines have a double carbon-nitrogen ring with four nitrogen atoms, while pyrimidine has a single carbon-nitrogen ring with two nitrogen atoms. Thus purines are bigger in size. Purines are numbered in the anticlockwise direction while pyrimidines are numbered in the clockwise direction. The atoms in the purine ring are numbered from 1 to 9, and in pyrimidine, they are numbered from 1 to 6.
The DNA and RNA in the body are made up of nucleotides. The main purines that can be found in the body are adenine (A) and guanine(G). The main pyrimidines that can be found in the body are cytosine (C), which is present in both DNA and RNA, and thymine(T), which is present only in DNA. In the case of RNA, instead of thymine, uracil (U) is present. The difference between the structures of thymine and cytosine is the presence of a methyl group in thymine and the absence of the methyl group in cytosine.
Other bases- Other than the aforementioned bases, DNA and RNA in a few cases can have other bases like 5-methylcytosine, N-acetylcysteine, N6-methyladenine, N6-dimethyladenine, pseudouracil, etc. These bases present in the nucleic acids help them recognize and bind to a specific base.
Sugars in nucleic acids- The main nucleic acids in the body are DNA and RNA. DNA stands for deoxyribonucleic acid and contains a sugar called D-deoxyribose. RNA stands for ribonucleic acid and contains a sugar called D-ribose. Both ribose and deoxyribose are pentose sugars and are numbered from 1′ to 5′. Deoxyribose sugar and ribose sugar both have similar structures, but in ribose, the C2 carbon atom is attached to a hydrogen and a hydroxyl group while in deoxyribose the C2 carbon atom is attached to two hydrogen groups.
The carbon atoms present in the sugars are numbered with a prime (‘) following their number to differentiate the numbering from the numbering of the nitrogenous bases.
A nucleoside is a pentose sugar bonded to a nitrogenous base. If the sugar present is ribose, it is a ribonucleoside, and if the sugar present is deoxyribose then deoxyribonucleosides are formed.
When a single phosphate moiety is bonded to a nucleoside, the term mononucleotide is used. Thus adenosine monophosphate
(AMP) contains adenine + ribose + phosphate. If the sugar is deoxyribose in place of ribose, it is denoted by dAMP.
The pentoses are bound to nitrogenous bases by beta-N-glycosidic bonds. The N9 of a purine ring binds with C1′ of a pentose sugar to form a covalent bond in the purine nucleoside.ln case of pyrimidine nucleoside, the glycosidic linkage is between N1 of pyrimidine and C1’ of a pentose.
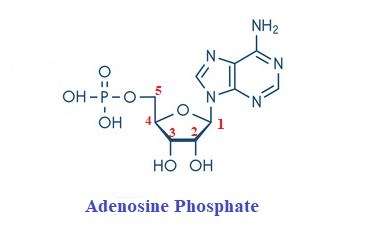
The phosphate bonds to the 3′ or 5′ carbon atom of the sugar through the hydroxyl group present just forming monophosphates.5′-Hydroxyl is the most commonly esterified so 5 ‘ is usually omitted while writing nucleotide names.
The phosphate group connects to one sugar by bridging the 5′-hydroxyl group and then binds to the 3′-hydroxyl group of the next sugar in the chain. These nucleoside linkages are called phosphodiester bonds. One nitrogenous is bonded to each sugar.
In DNA complementary bases (A-T & G-C) bind together. Thus a purine is always bonded to a pyrimidine. The A-T linkage is a double bond & G-C is a triple bond. They are bonded via hydrogen bonds.
