Nitrogen has an atomic number of 7 with an electronic configuration of 1s² 2s² 2p³. This means those 7 electrons are across these three orbitals.
| Element | Electron Configuration | Valence Electrons |
|---|---|---|
| Nitrogen (N) | 1s² 2s² 2p³ | 5 |

It is an element of the 15th group in the periodic table with a mass number of 14.01 u.
Of these seven electrons, two electrons are located in the inner orbit, akin to the carbon electron configuration.
The remaining five electrons are distributed in the outer orbit, as shown below.
Nitrogen ground state electron configuration
- The electronic configuration of nitrogen is 1S2 2S2 2P3.
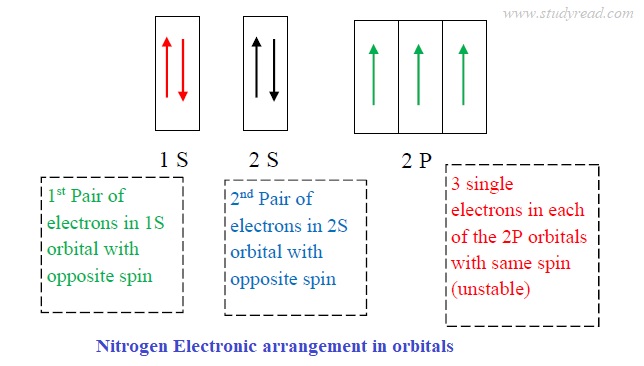
- The outermost orbital has a capacity of eight electrons.
- The 1S2 orbital is the innermost and has two stable electrons with the lowest energy.
- 2S2 orbital is in the outer shell and has two stable electrons with opposite spins.
- The nitrogen has a half-filled ‘P’ orbital, which is comparatively more stable.
- Thus, the ‘p‘ orbital is the outermost shell.
- Nitrogen needs to have a fulfilled ‘P’ orbital to achieve a stable gas configuration.
- The closest noble gas, neon (Ne), has the electronic configuration 1s2 2s2 2p6.
- Thus, the nitrogen needs to get 3 more electrons.
Nitrogen can form 3 covalent bonds with another atom of nitrogen to get a ground-state electron configuration.
Thus, it shares 3 electrons and completes the octet in both atoms.
Configuration in different forms of nitrogen.
Elemental nitrogen (N2-gas)
- Here, each atom of nitrogen is bonded to another atom via 3 bonds.
- Thus, a triple bond exists in this compound.
- One ‘S’ and a single ‘2P’ orbital combine to form ‘2 SP’ orbitals.
- One ‘sp’ orbital of nitrogen axially overlaps with the sp orbital of the other nitrogen to form a sigma bond.
- The lone pair of each nitrogen is contained in the other sp orbitals.
- There is a sideways overlap of p orbitals of nitrogen to form 2 pi bonds.
- So, each nitrogen shares 3 electrons and fills its outer orbitals.
Thus, it forms a linear structure that is SP hybridized.
Ammonia (NH3)
- The single 2s and the three 2p orbitals hybridize to give sp3 orbitals.
- Three sp3 orbitals are used to form bonds.
- Each bonded sp3 orbital overlaps with the s orbital of the hydrogen atom, thus forming an N-H bond.
- The non-bonding sp3 orbital contains a lone pair of electrons.
- Thus, nitrogen forms three bonds that share three electrons with hydrogen.
- So, it completes the octet, and the p orbital is filled.
- The hydrogen atoms also complete their duplet and completely fill their outer 1s orbital.
A tetrahedral structure is thus formed, and NH3 (ammonia) is sp3 hybridized.
Nitrosyl chloride (NOCl)
Here, the central atom is nitrogen, and it is doubly bonded to one oxygen atom and singly bonded to one chlorine atom.
- Thus, it shares three electrons and completes its outer shell.
- Here, the nitrogen is sp2 hybridized, having a triangular planar structure.
- Three sp2 orbitals are present: oxygen overlapping with one orbital, chlorine overlapping with another, and the last orbital occupied by a lone pair of electrons.
- Nitrogen also forms a pi bond with the oxygen to which it is bonded.
the image above is misleading. there are only 3 electrons in the p orbitals; adding another valence shell or displaying s&p would be more accurate. the way it is not would be more reminicant of florine