Carbohydrates are optically active poly-alcoholic aldehydes or ketones.
They contain the elements carbon, hydrogen, and oxygen in the ratio of 1:2:1.
These are biogenic and are found in both plants and animals.
Through food, one can avail large quantities of carbohydrates like sucrose, starch, cellulose, etc.
These carbohydrates are basically monomers and they form polymers to produce long-chain and complex carbohydrates.
The monomers of carbohydrates are either formed due to the breakdown of complex carbohydrates or by the synthesis in the body.
What is the monomer in carbohydrates?
A monomer is a molecule that can combine to form a polymer structure.
In carbohydrates, the molecules forming the backbone of the structure tend to join to form a polymer.
This monomer can combine with similar units to form complex or bulk forms like polysaccharides, cellulose, starch, dimers, tetramers, glycogen, etc.
Examples of carbohydrate monomers
The carbohydrate monomers are classified based on the number of carbons in their chemical structure. These include
Three carbon monosaccharides
This group has only one monomer.
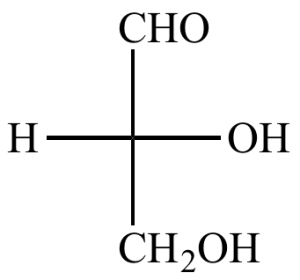
Example: Glycerol
- It is called glyceraldehyde.
- This is the three-carbon carbohydrate that has an aldehyde group in its structure.
Since three carbons are present, it is also called triose sugar.
This monomer is naturally found in oils and fats as part of fatty acid ester.
It is a soft viscous liquid that has a mild sweet taste.
Four carbon monomers group
This group has two monomers which have 4 carbon atoms in their structure.
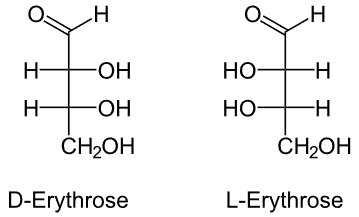
1. Erythrose
It is a 4-carbon monomer, i.e., a tetrose sugar.
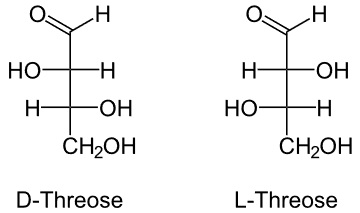
2. Threose
It is also 4 carbon monomer similar to erythrose in structure with a small variation.
Five-carbon carbohydrate monomers
Ribose sugar
- It is a 5-carbon monomer.
- A pentose sugar with many isomers.
- It is found widely in the Coenzymes, ATP, NADH, nucleic acids of living organisms.
- It makes up the sugar part of RNA (Ribonucleic acids), and its deoxy form of DNA.
So, ribose, a 5-carbon sugar, is one of the monomers of DNA and RNA.
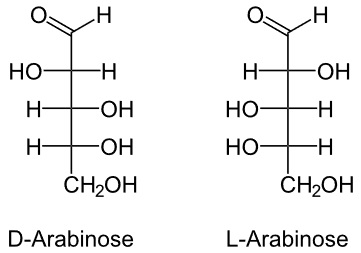
Example is Arabinose
- It is 5 carbon saccharide is an aldopentose.
- It can be obtained from guar gum, gum arabic, plum, cherry gums, and hydrolysis of vegetable matter.
- It is sweet in taste and has two isoforms, as below.
- But (L) is available in plenty in nature.
Another example is Xylose.
It is a five-carbon monomer obtained from wood.
It has two isomers and is an aldehyde, as in the image below.
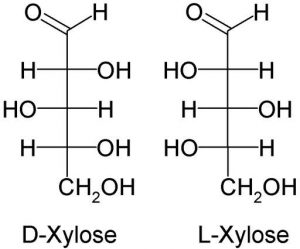
Notice the 3 central hydroxyl group (OH) arrangements. The center one differs from the other two in orientation.
It is a component of glycoproteins.
Another 5-carbon monomer is Lyxose.

Notice the central OH groups and compare them with xylose.
It is found in the heart muscle as a part of riboflavin.
It is rarely available in nature in free form.
Besides the above, there are also keto forms like ribulose, xylulose, etc.
Six carbon monomers
This group has monomers with carbons in them. These include 8 monomers like
1. Glucose

A 6-carbon carbohydrate and a hexose sugar.
It is one of the most abundant carbohydrates and sweet in taste.
2. Fructose
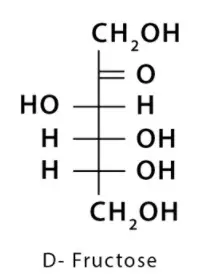
It is found in honey, fruit juice, etc.
It converts to glucose in the liver.
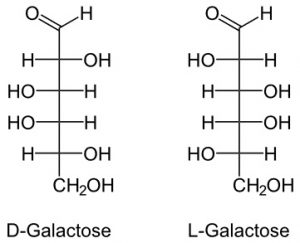
3. Galactose
It is also 6 member carbohydrate.
It is a milk sugar as it is found more in dairy products.
It is also found in gums and mucilage.
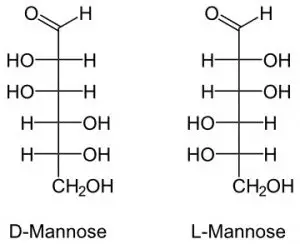
4. Mannose
It is a carbohydrate that controls protein quality. It is formed in the body from glucose.
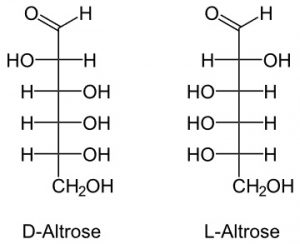
5. Altrose
It is a monomer found in a few bacteria.
6. Gulose
A 6-carbon monomer is found in bacteria, archaea, and a few eukaryotes.
It is sweet in taste.

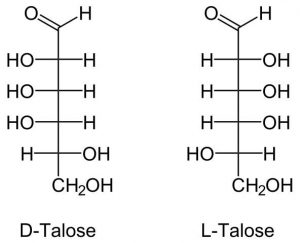
7. Talose
Another 6-member monomer that is not found naturally. But it can be made through a chemical process.

8. Heptose:
It is 7 carbon-containing carbohydrates and hence named monomer of carbohydrates heptose sugar.
Naturally, it is available as mannoheptulose in avocados, Fig.
Tests for carbohydrates help identify monomers of carbohydrates.
Frequently asked questions and answers.
What is the monomer of starch?
Glucose is the monomer of starch.
Is chitin a structural carbohydrate?
Yes, it forms an exoskeleton in insects, crustaceans, and even mushrooms.