Monoatomic elements are those elements that exist as a single atom physically in nature.
For example, gases like hydrogen, and oxygen exist in nature as molecules made of 2 atoms as H2 and O2.
This means the element hydrogen exists as two atoms of hydrogen combined as H-H.
And, oxygen exists as two conjugated atoms of oxygen as O-O.
Hence, oxygen and hydrogen are called diatomic elements as they exist as two atoms in combined form in nature.
While you try to separate them as H and O instead of H2 and O2 they will be highly reactive and tend to combine back to form stable molecules.
Hence, by nature, they exist as a combination of two atoms due to the instability of their monoatomic forms.
Whereas monoatomic elements are those substances that have one atom existing freely in nature without being conjugated chemically with another atom.
This means these elements have single atoms that are highly stable naturally.
Noble gases as monatomic elements
All the noble gases of the periodic table are called monoatomic elements. They exist as stable gases in nature. These include
- Helium (He)
- Neon (Ne)
- Argon (Ar)
- Krypton (Kr)
- Xenon (Xe)
- Radon (Rn)
Helium (He)
It is the second element in the periodic table after hydrogen and is a highly stable inert gas.
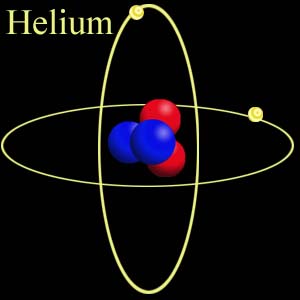
It is chemically represented as He.
As seen in the picture above, it has two of its outer shells filled with electrons and does not need electrons from other atoms for stability.
Hence, the atom is highly stable and exists individually and not in pairs. Its atomic number is 2 and its mass number is 4.003.
Neon (NE)
This is the next noble gas on the list of stable gases. Like Helium, it also has a full valence outer shell with eight electrons.
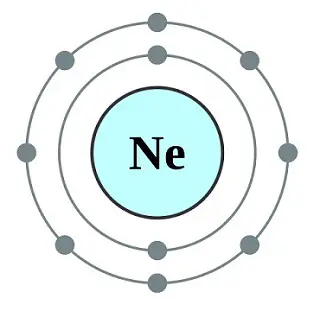
The monoatomic elements exist as a single atom as Ne with an atomic number of 10 and an atomic mass of 20.1797.
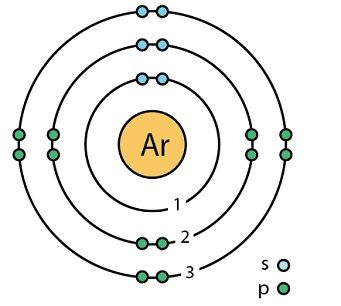
Argon (Ar)
Another stable gas with 8 electrons in the outer shell.
It is also a mono-atomic represented chemically as Ar.
It is the third of noble gases.
Krypton (Kr)
It has an atomic number of 36 and a mass number of 83.798.
Represented as Kr chemically, it is another noble gas in the monoatomic form.
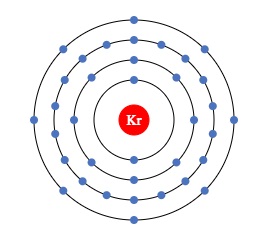
It has also fully filled and paired valence electrons making it a highly stable atom in spite of being single.
Xenon (Xe)
It is another noble gas element with a single atom in the structure.
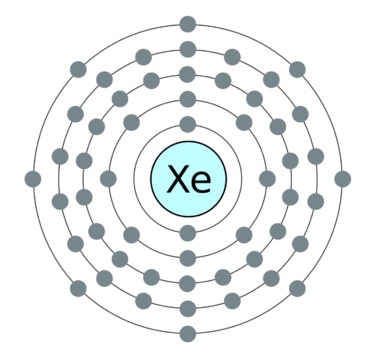
It has atomic number 54 and an atomic weight of 131.293.
It is also highly stable due to completely occupied outer orbitals with paired electrons. The element is a single atom represented as Xe.
Radon (Rn)
Radon is also a monoatomic structured element. It exists as a single atom of Rn.

The atomic number is 86 and the atomic mass of 222.
Besides these inert gases, there are also heavy metals that can exist in monoatomic form with different physical and chemical properties when their lattice is disturbed. These include
Gold (79), Silver (47), Platinum (78), Palladium (46), Rhodium (45), Ruthenium (44), Osmium (76), Iridium (77) etc.
In nature metal atoms are monoatomic but they are bound by metallic bonds in a lattice such that more atoms of the same element are kept together. But there are no chemical bonds between these atoms and hence can easily break up to form monoatoms by a certain force.
Even Hydrogen and other chemicals can also be found in the monoatomic form in space at specific temperatures.
Does monoatomic gold have any antigravitational or superconductive attributes?