Ion exchange chromatography is an interesting type of column chromatography.
As you know, Chromatography is a process of the separation of molecules from a mixture.
This separation is done based on the differences in the sample’s adsorption coefficient or partition coefficient with the stationary phase.
Whereas in ion-exchange chromatography, segregation of molecules occurs based on ion displacement theory. This means a more potent ion displaces a weaker ion.
This can be seen by an example.
When Magnesium hydroxide is allowed to react with hydrochloric acid, it forms a salt of Magnesium Chloride and water.
MgOH + 2HCl —————————> MgCl2 + H2O
In the above reaction, more reactive Cl- replaces less reactive ion OH- to form magnesium chloride.
Ion Exchange chromatography Principle
The charged molecules in the sample are separated by the electrostatic forces of attraction when they pass through the ionic resin at particular pH and temperature.
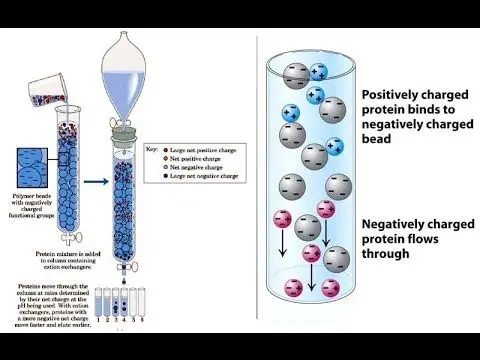
The separation occurs by reversible exchange of ions between the ions present in the solution and those present in the ion exchange resin.
The separation of molecules from the mixture depends on the type of ion exchange resin used.
- For example,
Cation exchange resins are employed to retain cations in the column.

And anion exchange resins are used to keep anions into the column from the sample.

Thus the process of separation would be like the one shown in the video below.
As the desired ionic molecules are stuck in the resin, the column is allowed to exhaust (flow) till all the other ions are flushed out.
Then the desired ions stuck in the resin are allowed to flow out by passing a more reactive mobile phase through the column.
If cations (+) are present in the column, a strong base is moved into the column and vice versa.
Many drugs, enzymes, proteins, and other pharmaceutical products are either acidic or basic in nature.
Hence this method can be used to separate and purify these charged compounds.
Types of ion exchange resins
Ionic resins are of two types as
Different types of resins are used for the separation of different materials.
Chemically they are classified as
- Cation exchange resin
- Anion exchange resin.
| Resin | Nature | Functional group | pH range | Used for |
|---|---|---|---|---|
| Cation | Strong | sulfonated (SO3H) | 1-14 | Separation of inorganics like lanthanides and cations like amino acids, Vitamin-B’s |
| Weak | carboxylic (COOH), hydroxyl (OH), phospho (PO3H) | 5-14 | For separation of antibiotics, organic alkalies and other cations. | |
| Anion | Strong | quarternary and tertiary ammonium (N+R3, NR2 | 0-12 | Separation of alkaloids, fatty acids |
| Weak | Polyamines, phenols (NH2, C6H5OH) | 0-9 | Separation of anions like complexes and vitamins. |
Based on the structure, they are of the following types like
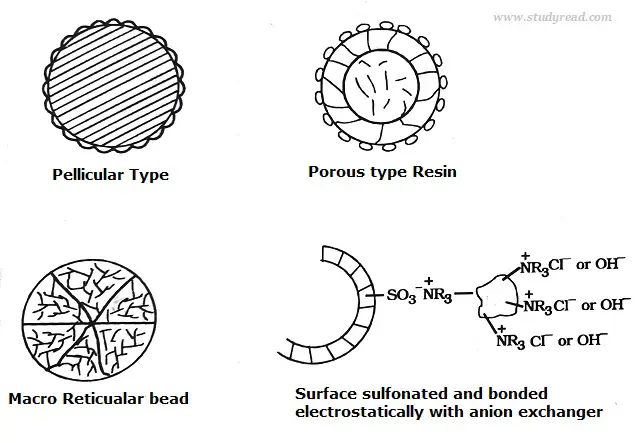
- Pellicular type with a particle size of 30 to 40microns and 1 to 2 microns thickness.
- Porous resin is highly efficient, with a size of 5 to 10 microns.
- Macroreticular resin which less efficient.
- Surface Sulfonated type.
Ion exchange chromatography procedure
This involves following steps like
- Selection of suitable ion exchange resin
- Preparation of mobile phase
- Packing the resin into the column
- Development of chromatogram for elution.
Select a suitable column made of polymers or steel with dimensions of length: diameter of 20:1 to 100:1 for better separation efficiency.
Mobile phase solvents used in this chromatography or mostly buffers, acids, or bases.
The buffers can be acetate buffer, borate buffer, phosphate buffer, or phthalate buffer. At the same time, acids can be 0.1N Hydrochloric acid and bases like 0.1 or 1N sodium hydroxide.
Mix the resin with the mobile phase and pack it into the column without any gaps.
Dissolve the sample under test into a sufficient quantity of mobile phase.
Introduce the whole sample solution from the top of the column.
The chromatogram is developed by introducing the mobile phase by the isocratic or gradient method of elution.
The isocratic method involves the use of the same solvent as the mobile phase throughout the development.
While the gradient method involves using a mixture of solvents as a mobile phase, initially, one solvents ratio is used and altered as the development proceeds.
Applications of ion-exchange chromatography.
Ion exchange chromatography is prominently used as preparatory chromatography to isolate the desired compound from the mixture.
Hence the applications are meant to obtain pure compounds.
a) For deionization and softening of water
Hard water is one of the common problems in most parts of the world.
This is especially seen in tubes or groundwater. Due to the decline in groundwater levels, the problem is getting severe.
So, the hard water is made soft for drinking purposes.
The calcium and other salts present in water are removed by this method.
b) Purification of a solution to keep them ion-free.
c) In biochemistry for separation of drugs and metabolites from blood, urine, etc. This finds application in clinical diagnosis.
d) Used for separation of organic compound mixtures of proteins, carbohydrates, nucleotides, etc.
e) For purification of enzymes after extracting from the tissues.
Disadvantages of ion-exchange chromatography
- Organic solvents cannot be used for the separation
- The choice of samples is limited as only ionic forms can be isolated.
- Requires more solvents and resins for each separation making it expensive.
Also, see Gel Permeation Chromatography.
What other method of water demineralization.