Oils and fats are routine ingredients we use as part of our food. To check their quality, they are analyzed by
1. Iodine value
2. Saponification value
3. Hydroxyl value
4. Acetyl value
5. Acid value
6. Ester value
7. Polenske value
8. Kries test
There are many tests to assess their quality and chemistry.
Oils are naturally occurring products from plants and animals.
Ex: Cod liver oil, omega fatty oils, Arachis oil, mustard oil, etc.
They are the reserve food material with good nutrition value.
Physically they are viscous or semi-solids by nature.
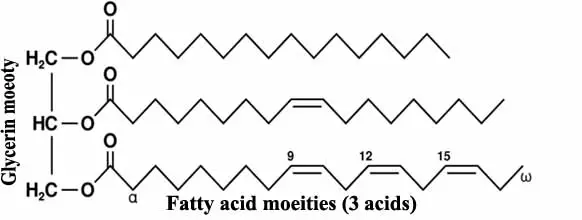
Chemically they are esters of fatty acid and glycerin to form triglyceride esters.
These fatty acids are either of saturated type or unsaturated type.
When oils and fats are kept in atmospheric air for a long time, develop an unpleasant odor.
This is because of a chemical process called rancidification or rancidity. The fatty acids react with oxygen from the air to form aldehydes and ketones. So the development of disagreeable odor. This can render the oil useless for consumption.
The extent of rancidity depends on the amount of exposure and also the extent of unsaturation.
Also, the oils which we buy from the market can be adulterated. There may be other impurities besides. To check these issues, we go for a qualitative analysis of oils.
To asses the quality of oils and fats, one has to take into account the physical and chemical properties of oils and fats.
Analysis of fixed oils and fats.
In general oils and fats can be identified and evaluated for their quality and impurity if any by physical and also chemical constants. Physical constant includes a refractive index, viscosity, specific gravity, etc.
On the other hand, one can perform chemical methods of analysis. These chemical methods check for the type of fatty acid, the extent of unsaturation and rancidity if any.
Iodine value: It is defended as the weight of iodine absorbed by 100 parts per weight of the given sample of oil or fat. It gives a measure of the extent of unsaturation in oils. Oils which are unsaturated take up iodine to add up the bonds using iodine. The more the iodine value, the higher is unsaturation and thereby chances of rancidity.
Saponification value: It is the number of milligrams of potassium hydroxide (KOH) required to neutralize the fatty acids resulting from hydrolysis of 1gm of given oil or fat.
Hydroxyl value: It is the number of milligrams of KOH required to neutralize the acetic acid which can combine by 1gm of oil or fat by acetylation process.
Acetyl value: It is defined as the number of milligrams of KOH required to neutralize the acetic acid obtained when 1gm of sample acetylates oil is saponified. Most oils have low acetyl value like 3-15, unlike castor oil, which has a value of 150.
Acid value: It is defined as the number of milligrams of potassium hydroxide required to neutralize the free acids present in 1gm of fat or oil. During rancidity, acid is released. This acid value indicates the extent of rancidity.
Ester value: It is the number of milligrams of KOH required to combine with fatty acids present in ester form (triglyceride form) in a gram of oil or fat.
Difference between Saponification value and Acid value gives Ester value.
Polenske value: It is primarily used for the analysis of fats. It is defined as the number of milliliters of KOH required to neutralize water-insoluble steam liberated by hydrolysis of 5 grams of fat.
Kries test: This is also called rancidity index. When the oil is kept for a long time, it gets rancid due to atmospheric oxygen. The oil undergoes oxidation to form various forms of aldehydes and ketones, making it unsuitable to consume and also produce disagreeable odor.
Here malonaldehyde and epihydrin formed due to rancidity are identified on treating with phloroglucinol. On reacting with oxidized fat or oil, phloroglucinol produces a red color.
For testing, one uses a fixed amount of oil like 1gram taken into a beaker with some suitable indicator to indicate endpoint and titrate it by using 0.1N KOH dropping from a burette above. This is a type of acid-base titration.