Gas chromatography is one in which an inert gas is used as a mobile phase to separate components of a sample.
Based on the nature of the stationary phase, this chromatography is of two types, like
- Gas-liquid chromatography (GLC) and
- Gas-solid chromatography (GSC).
Of them, GLC is widely used, so our entire discussion would be related to it.
Principle of Gas Chromatography
- The principle in gas chromatography involves the separation of volatile components of a sample between the mobile gaseous phase and stationary liquid phase.
- The solubility of a substance in between the gaseous mobile phase and stationary liquid phase is based on their partition coefficient.
- The components of the sample that are partitioned into the gas phase come out first, while others come later.
Thus, the components of the sample are separated based on the principle of partition chromatography.
The stationary phase is a liquid layer spread over a stationary phase.
While the mobile phase is an inert and stable gas.
Hence the name “Gas-Liquid chromatography (GLC).”
This type of chromatography was primarily designed to evaluate volatile compounds like fatty acids, essential oils, etc.
However, with few modifications, it can be used on various samples.
The system is quite expensive, cumbersome, and also has delicate instrumentation. Even the instrument’s maintenance and operating costs are very high.
But still, gas chromatography is an important tool in analytical chemistry, especially in the medicinal field.

How gas chromatography works:
- The gas is set to flow at a constant rate from the cylinder onto the liquid layer impregnated on a solid support in a column.
- The sample is injected into the injection point and is carried by the mobile gas into the column.
- Inside the column, the components get separated by the differential partition between the mobile phase gas and stationary phase liquid.
- The component that partitions into gas comes out of the column first and is detected by the detector.
- The one partitioned into the liquid phase comes out later and is also detected. The recordings are displayed on computer software.
- From these peaks, one can identify the components and their concentration.
Gas chromatography System

Gas chromatography instrumentation and requirements
The gas chromatography apparatus has the following
Gas cylinder
The mobile phase is a gas, as discussed before and it is held in compressed form in a metallic cylinder.
The mobile phase gas in a cylinder
The gas used as a mobile phase should be inert and non-reactive in nature.
Monoatomic element gases like helium or other non-reactive gases like nitrogen & hydrogen.
The most commonly used gas is helium.
The carrier gas is kept in a metallic cylinder and outflow is controlled by a regulator.
The flow regulator
It lets the gas from the cylinder pass at a fixed rate. Any alteration in the rate of gas flow leads to improper measurement and analysis.
The gas is passed at a fixed rate through a pressure gauge from the gas carrier cylinder.
This gauge indicates the speed of the flow of gas into the column.
The injection system
This is present before the column yet inside the thermal chamber, to load the sample under analysis into the system.
The column is for gas chromatography.
The gas chromatography column is usually long (a few meters, like 3 to 6 meters) and coiled for accommodation in a small thermal chamber. The column is mostly made of steel or glass.
The GC columns are of three types, viz.
i) Packed column.
This is a column into which solid beads are packed. This column has advantages like efficient separation and precise readings.
ii) Tubular column.
Here is a stainless steel hollow tube; a thin layer of liquid is coated onto the inner wall of the tube to act as a stationary phase.
This column is designed to offer the least resistance to the gas flow.
iii) Support-coated tubular column.
Here in the stainless steel column, a thin solid layer is coated onto which a thin liquid stationary phase is present.
The Detector
It is another vital component of gas chromatography equipment.
It helps to detect the isolated components coming out of the column by the flow of gas.
Due to this, there are peaks formed in the recorder, which help in the identification and quantification of the sample.
There are four types of GC detectors
i) Thermal conductivity detector.
Here, two columns have a conducting wire in between. The gas is allowed to pass directly through the two columns of detectors, i.e., to one the effluent from the gas chromatography column and to another gas from the gas cylinder. Since the temperature of both gases is the same, the thermal conduction is constant.

When the sample is injected into the gas chromatography column, the effluent gas carries the sample components into the detector column.
Since effluent gas is mixed with sample components, there results in a difference in thermal conductivity from prior recording. This difference in conductivity is specific to the component analyzed. This is recorded for further comparison and identification of the components and their quantity.
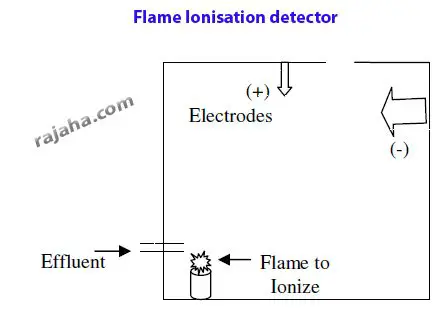
ii) Flame ionization detectors
Here, the effluent sample components are ionized by exposure to flame in a chamber.
These ions rise upwards and are attracted toward an anode or cathode based on their charge.
When they impinge on the electrodes, the current is passed, which is recorded. The strength and intensity of the current generated depend on the sample and are specific.
iii) Argon ionization detector
These detectors are similar to flame ionization detectors, with the only difference being that argon-ion gas is used to ionize the sample molecules.
The argon ions are obtained by reacting argon gas with radioactive elements.
Once argon ionizes, it tries to get back to the stable state by either taking or giving electrons from the sample components, thus making sample molecules into ions for detection.
iv) Electron capture detector, etc.
The computer
This system records the analyzed readings.
This is connected with the detector and hence records the detector changes in reference to the flow of separated components from the exit of the column.
The record is called a gas chromatograph.
The thermal chamber
It helps to fix or maintain a fixed temperature.
Pre-column treatment
This is a technique used to modify the constitution of the sample before it enters the column.
The low-volatile compounds are chemically modified into the highly volatile compound.
This helps in better separation and also the identification of the compounds.
Post-column treatment
This is intended to alter the components coming out of the column to be easily detected by the detector.
The inclusion of pre-column and post-column treatment or derivatization helps to increase the range of the compounds that can be estimated by gas chromatography.
The compounds with low volatility can be determined by making them volatile by pre-column treatment.
And those compounds with poor detection by the detector can be altered for easy detection by the detector.
The entire system should be placed inside a chromatography lab. This should be well equipped with proper lighting, space, and air conditioning.
See the applications of gas chromatography.

As a further improvement in GC, the gas chromatography apparatus is fixed with a Mass spectroscopy system (GC-ms) to analyze components regarding their mass better.
nice explantion
Very good site to know more information about any topic
Supper
Good work
Very great information
I also want to cite your article can i also have your details?
Very Good
I want to write your resource as one of my refrences, but I could not know your name
MLA citation:
Ahmed! sent you mail with details…
please how do i cite your work as a reference. thanks
@Ian! you can use ranga reddy n as author name and url of this page as reference with date of 2012.