Enzymes are biocatalysts that enhance the speed of biochemical reactions without themselves undergoing any change.
They are biomolecules that are synthesized by living cells.
They are protein in nature (except – RNA, which acts as a ribozyme) and are colloidal and thermolabile (destroyed by heat) in nature.
They are reaction specific and are meant for one particular type of process.
Almost all biological reactions in the body are carried out in the presence of enzymes. This increases the optimal output of the reaction.
The temperature of the body is also regulated at a range where the biological enzymes are maximally active.
At too low temperatures, the thermodynamic energy is too less, so the substrate and the enzyme do not interact enough to produce products at a sufficient rate.
In contrast, when the temperature is too high, the enzymes undergo chemical denaturation and become inactive. Thus high temperature may be a form of physical inhibition.
Every enzyme shows optimal activity at a certain pH. This can be considered a form of chemical inhibition.
An enzyme inhibitor is a substance that binds with the enzyme and brings about a decrease in the catalytic activity of that enzyme.
Types of Enzyme inhibition
This can be classified into the following types as
1. Reversible inhibition
- Competitive inhibition
- Noncompetitive inhibition
2. Irreversible inhibition
3. Allosteric inhibition
4. Feedback inhibition
Reversible Inhibition
The inhibitor binds to an enzyme via non-covalent transient bonds. Due to this, the enzyme inhibition gets reversed if the inhibitor is removed. This is also called the equilibrium type of inhibition.
It is of two types.
Competitive inhibition
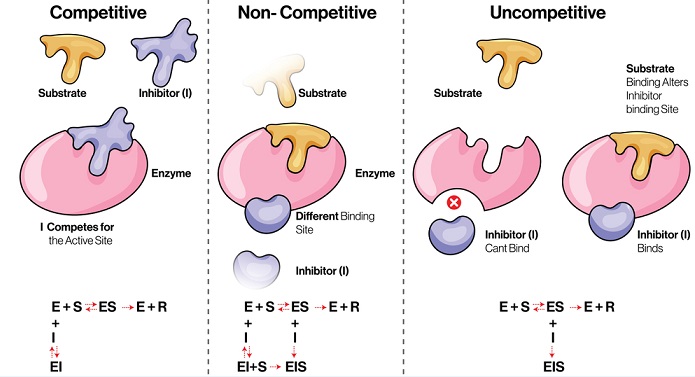
Here the inhibitor molecule is structurally similar to the specific substrate of the enzyme.
So at the active site, the inhibitor competes with the substrate and fits into the active site of the enzyme instead.
Thus, the enzyme is not available for the substrate to bind until the competitive inhibitor leaves the active site.
The binding of a substrate to the active site produces a product.
Whereas binding of the inhibitor to the active site of the enzyme produces either no reaction or a non-functional product is formed.
The relative concentration of the substrate and the inhibitor and their respective affinity with the enzyme determines the degree of competitive inhibition.
High substrate concentration displaces the inhibitor from the active site. Thus the affinity has decreased (Km has increased), but the maximum velocity remains the same.
Some therapeutic uses of competitive inhibition are
A. In the case of methanol poisoning, ethanol is administered as an antidote.
This is because methanol is metabolized to formaldehyde by the enzyme alcohol dehydrogenase (ADH).
This formaldehyde is further metabolized to formic acids, which are toxic and cause adverse symptoms.
Ethanol competes with methanol for the same enzyme ADH and prevents the further formation of formaldehyde.
Thus, ethanol can be used in the treatment of methanol poisoning as it reduces the poisonous effects of methanol by competitive inhibition.
B. Xanthine and Hypoxanthine are the substrates for the enzyme xanthine oxidase, which converts them to uric acid.
Excess uric acid in the body forms crystal deposits in the joints causing gout. The drug allopurinol acts as a competitive inhibitor of the enzyme and helps in the treatment.
C. HMG CoA reductase enzyme converts HMG CoA in the body to cholesterol. In cases of hypercholesterolemia, competitive inhibitors like lovastatin are used.
Noncompetitive inhibition
In this reaction, the inhibitor binds to the enzyme at a point other than the active. This impairs the enzyme function.
The inhibitor has no structural similarity with the substrate.
There is a strong affinity for the inhibitor to bind at the second site of the enzyme, so it does not interfere with the enzyme-substrate binding.
The inhibitor generally binds with the enzyme as well as the Enzyme Substrate complex.
But the catalysis is prevented due to a change in the enzyme conformation.
The affinity of substrate and enzyme is unchanged, but the maximum velocity of the reaction is lessened.
Examples: Heavy metal ions like Pb2+, Ag+, Hg2+, etc., can bind with cysteinyl sulfhydryl groups of enzymes, thus non-competitively inhibiting the enzymes.
Irreversible Inhibition
Here the inhibitors form strong covalent bonds with the enzymes and irreversibly inactivate them. This is also called a non-equilibrium type of inhibition.
Usually, such inhibitors are known as poisons or toxins because it is difficult to control their activity as they produce intensive effects.
Some therapeutic uses of irreversible inhibition are
A. Di-isopropyl fluorophosphate (DFP) is a nerve gas that irreversibly binds with enzymes containing serine at the active site (serine proteases, acetylcholine esterase). It was developed by the Germans during Second World War.
B. Organophosphates are irreversible inhibitors of acetylcholine esterase. This enzyme is essential for nerve signal transmission. So organophosphate poisoning results in muscle paralysis and eventually death.
C. Iodoacetate is an irreversible inhibitor of enzymes like papain and glyceraldehyde 3-phosphate dehydrogenase.
D. Penicillin is an irreversible inhibitor of serine-containing enzymes and blocks bacterial cell wall synthesis. Thus it is a potent antibiotic.
Disulfiram is a drug that irreversibly inhibits the enzyme aldehyde dehydrogenase, which leads to acetaldehyde accumulation in the body. This makes the person sick and makes him avoid further intake of alcohol.
Suicide inhibition
This is a special form of irreversible inhibition. Here the original inhibitor loses its structure as it is acted upon by the same enzyme that it inhibits.
It gets converted to a more potent form.
The newly formed inhibitor binds irreversibly with the enzyme, whereas the original inhibitor would have bound reversibly.
Thus enzyme inhibition becomes stronger and maximum velocity cannot be reached.
Allopurinol is an example of suicide inhibition (used in the treatment of gout).
Allopurinol is an inhibitor of xanthine oxidase. Xanthine oxidase converts Allopurinol to alloxanthine, a more effective inhibitor of the enzyme.
Certain purine and pyrimidine analogs used in cancer therapy operate on the principle of suicide inhibition.
The drug S-fluorouracil gets converted to fluoro-deoxy uridylate, which inhibits the enzyme thymidylate synthase. This inhibition reduces nucleotide synthesis.
So now, enough DNA and RNA are not formed and the cancer cells cannot proliferate.
Allosteric Inhibition
Some enzymes possess additional sites other than active sites, known as allosteric sites.
Such enzymes are known as allosteric enzymes and the sites are specific for every enzyme.
Most allosteric enzymes are oligomeric in structure. Their subunits may be identical (homopolymeric) or different (heteropolymeric).
When the effector molecule binds at the allosteric site, there is a conformational change in the enzyme.
The effector molecule may be an inducer or inhibitor of the enzyme-substrate interaction.
In the case of allosteric inhibition, the effector molecule binding induces a conformational change that does not allow the substrate to bind to the enzyme.
Allosteric enzymes exist in two conformational states-the, T (tense or taut) and R (relaxed).
The T and R states are in equilibrium with each other. Allosteric inhibitors favor the Taut state while activators and substrates favor the Relaxed state.
Some enzymes inhibited by allosteric mechanisms are
A. The enzyme hexokinase is inhibited by glucose-6-phosphate.
B. The enzymes phosphofructokinase and isocitrate dehydrogenase is inhibited by ATP.
C. Enzyme Acetyl CoA carboxylase is inhibited by palmitate.
Feedback inhibition

Here the end-product feedback regulates its own formation.
This is a mechanism of enzyme inhibition where the product formed from the biochemical reaction inhibits further enzyme action.
This occurs when the concentration of the product reaches certain levels. The product reaches and binds to the enzyme and brings conformational changes in it, leading to inhibition of its catalytic functions.
i love this