Chromatography is a chemical analysis technique that relies on the separation of components of a mixture.
It is derived from the Greek terms “chromo= color & gram=bands.”
Hence, one can see the formation of colored bands in column chromatography.
These bands are indicative of different components in the sample.
Chromatography definition
“Chromatography is an analytical technique wherein a sample mixture under test is separated into different components.”
This is both a qualitative and a quantitative analytical method. One can know the individual components of the sample as well as determine their concentration.
These separated components are later identified and quantified.
Principle of chromatography
Chromatography relies on the principle of separation of compounds.
The sample components get separated under the influence of a mobile phase while moving through the stationary phase.
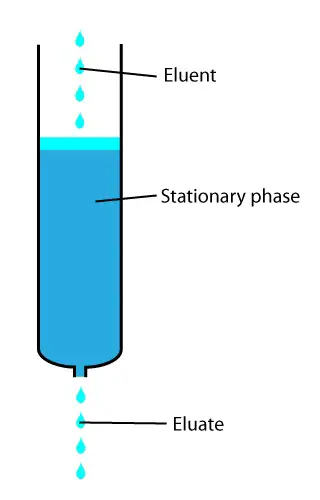
The differential separation occurs due to varying affinities of the compounds for the stationary and mobile phases.
The component with greater affinity for mobile phases moves faster, while the component with greater affinity to stationary mobile phase moves slower.
After this separation, the compounds are identified by suitable detection methods.
The differences in affinities arise due to relative adsorption or partition coefficient between components towards both phases.
Adsorption varies due to the polarity of components towards the stationary phase. If both the stationary phase and components in the sample are polar, the rate of travel for the polar component is slow and, hence, gets separated from the rest of the sample and is passed out of the column last. Similarly, if both the stationary phase and component in the sample are non-polar, then the non-polar component comes out last due to the slow rate of travel in the column under the influence of the mobile phase. So, the stationary phase and mobile phase are always opposite. I.e., if the stationary phase is polar, the mobile phase used is non-polar and vice-versa.
Ex: In a sample of a mixture of lipids and proteins. Lipids are non-polar, and proteins are polar in nature. Suppose here Sand, which is polar, is used as the stationary phase, and non-aqueous solvents like Hexane or Acetone, which are non-polar in nature, are used as the mobile phase. During separation, lipids come out of the column first, while proteins come out later due to their higher adsorption to the sand stationary phase.
Partition varies due to the solubility of components in different liquids. So here, both the mobile phase and stationary phase must be liquids. The stationary phase liquid is in the form of a thin layer of film on a hard background in the column. So when a sample mixture has components of differences in solubility in different liquids of the same nature, i.e., either polar or non-polar, they get separated into two liquid phases, i.e., mobile phase and stationary phase, based on their partition coefficient in between two liquids.

Ex: Iodine in between water and trichloromethane. Though iodine is soluble in both, it is non-polar in nature and, hence, is more soluble in trichloromethane than in water. So, most of the iodine separates into it, and a tiny amount stays in the water.
Chromatography techniques:
There are different techniques in chromatography. For details, go through Types of Chromatography, But common technical requirements for all the types include
1. Stationary phase: The stationary phase is one which stays motionless and allows the sample to move over it. This phase can be solid or liquid. If it is a solid stationary phase, it should have particles of uniform size and shape. Moreover, their shape should be preferably spherical. If a liquid is used as the stationary phase, the liquid is spread as a uniform layer on the solid background. The chromatography columns house the stationary phases in all types of chromatography except on paper and thin-layer chromatography, as they do not have a column.
2. The mobile phase: This is the chromatography liquid, and it helps the sample move over the stationary phase. The mobile phase used is a liquid or gas, and it should be free of particle matter and other impurities. Technically, the mobile phase should have the opposite polarity to that of stationary phase material. I.e., if the stationary phase is of polar in nature, then the mobile phase has to be non-polar and vice-versa. In normal-phase chromatography, the mobile phase is non-polar in nature, while in the reverse phase, the mobile phase is polar in nature.
3. Flow rate: the rate of flow of the mobile phase over the stationary phase is always kept constant. It should be uniform over the entire period of the experiment for reliable results. The high rate will help with faster separation, but the band can be very close. If the rate of flow is slow, it will be time-consuming and also require a more mobile phase.
4. Temperature: The temperature of the experimental chamber or chromatography lab is kept uniform. Alteration in temperature can alter the flow rate, the state of the mobile phase, and also the detector efficiency.
5. Treatment of sample: Samples sometimes need to be treated for better separation or detection by the detector. In such cases, the sample is chemically altered before or after the process of separation. This is termed as pre-column or post-column derivation. This is especially followed in gas chromatography for some solvents.
6. Other techniques like ascending, descending, and radial modes of development of chromatograms are followed.
7. The test sample or standard sample used in the analysis is moistened with the solvent, preferably the mobile phase, and it should be soft, powder form, homogeneous, or pulpy but not hard.
8. The preparative or analytic technique is employed. For preparative HPLC, the sample quantity is larger and is aimed to get the pure compound. In the analytic technique, samples are just analyzed for identification of the sample and also the determination of its quantity.
Also, see the Difference between Chromatography and Electrophoresis
Chromatography Uses :
Chromatography is one of the widely used analytic techniques in many industries.
- It is used in the food industry to check for permissible limits of ingredients.
- It is used in clinical diagnosis to see the levels of the drug in the blood at different time intervals (Kinetics & bio-availability study)
- In scientific research, to check the biological substances and their quantity in the extracts.
- In quality control of drugs, in pharmaceutical industries. Once the drug formulation is ready, it is evaluated for quantities of different ingredients. This is done to comply with USP and other pharmacopeial standards.
- It is specifically used to identify and measure the molecules having similar chemistry, optical absorbance, etc.
References:
very helpful
I like the way things have been explained.
This helped a lot.
Thank you.
this page is very helpful.
Thank you so much
It is very simple language to learn THANKS
Thank you for this. It is very easy to understand.
Please say more and more
Nice page …easy to understand
Thank you because its easy to understand thank you ones again.
Its really simple to understand thanks.
nice job..
oh wonderful,it is precise, brief and rich in content.
A veru good page. Explained all the terms n principle of chromatography for beginners
realy, nice job
The definition is not clear to me
This is a great page
I don’t like this site. I do not understand the definition.
@Laceigh Richeme! For the ease of understanding, we have updated the definition again. Check out.
Even layman can understand this type of simple language
i love this simple and clear explanation