The different chemical species, like atoms, ions, and molecules, exist together by bonds.
A chemical bond is a lasting attraction between two chemical species.
Depending on the specific type of bond and the particular species’ nature, the bonds may be strong or weak.
Chemical species usually bond either.
- to fill their electron orbitals and shells or
- due to the attraction between two oppositely charged ions or
- the electronegativity difference between two atoms in separate molecules.
The amount of energy required to break one mole of a specific bond is known as the bond enthalpy or bond energy of that bond.
Chemical bonds can be classified into two types as
Primary (strong) bonds-
These are the bonds that are strong and difficult to break. These include like
- Covalent,
- Ionic and
- Metallic bonds.
Secondary (weak) bonds
These bonds are weaker comparatively and are temporary bonds like
- London dispersion forces,
- Dipole-dipole force, and
- Hydrogen bonding.
Covalent Bond

This type of bond is also called the molecular bond. In this bond, the electrons are shared among the two atoms.
Thus each covalent bond comprises a pair of electrons, one electron belonging to each chemical species.
This type of bond is usually formed between two nonmetal atoms with similar or almost the same electronegativity values.
This sharing of electrons usually occurs such that the atoms involved can fill their outer orbitals to achieve a stable noble gas configuration.
The electron pair that take part in the covalent bond is called a bonding pair/ shared pair. The electron pairs not taking part in the bond are known as lone pairs.
The bond pair and the bond are represented as a straight line between the two atoms. Each lone pair is represented as two dots on top of the atom that it belongs to.
Covalent bonds have interactions and overlap the sigma and pi orbitals in the two atoms. Thus we can have a covalent single, double, and triple bond.
Covalent bonds are of two types as
- Nonpolar covalent bond and
- polar covalent bond.
A non-polar bond is one where the two atoms share the atoms equally; it is known as a non-polar/pure covalent bond.

This occurs when the atoms participating in the bond are the same.
This is the case in hydrogen gas ( H2), oxygen gas( O2), nitrogen gas (N2), etc.
A polar covalent bond is present in compounds or radicals where the two atoms have varying electronegativities like NH3 or CH4.
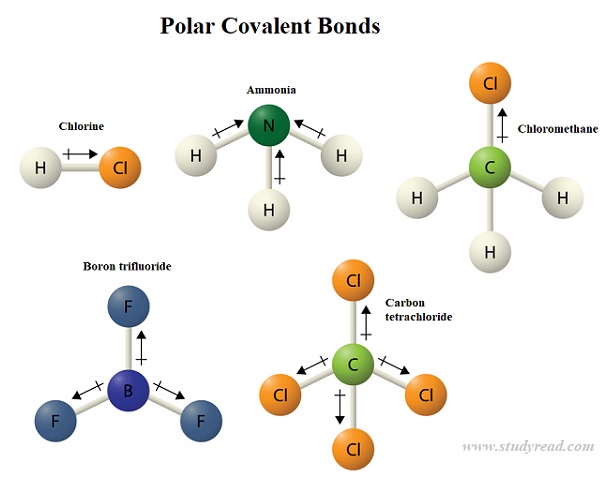
In this case, the electrons are pulled more towards one atom ( the more electronegative one) than the other.
Thus there is unequal sharing. More the electronegativity difference between the two atoms, the more polar the bond is.
Ionic Bond
The ionic bonds are formed due to an electrostatic force of attraction between two oppositely charged ions.
This bond is usually formed between a metal and a nonmetal. Before the electrostatic attraction, there is a total transfer of valence electrons from one atom to another atom.
This goes as ⇒ Na+ + Cl– → NaCl.
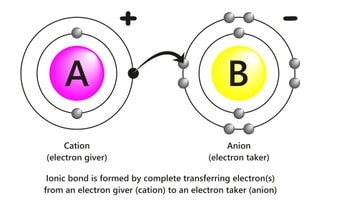
The first atom (usually a metal electropositive in nature) loses electrons to achieve the closest noble gas configuration, fulfill the octet rule, or have completely filled outer orbitals.
This loss of electrons is facilitated by ionization energy and makes the ion stable. The second atom (usually a nonmetal, which is electronegative in nature) accepts electrons to get the closest noble gas configuration, fulfill the octet rule, or have completely filled outer orbitals.
This is facilitated by electron gain enthalpy. The oppositely charged ions get attracted due to electrostatic forces, and an ionic bond is formed between them, releasing energy.
Ionic compounds easily dissociate into separate ions in water.
Common examples of ionic compounds are table salt (sodium chloride-NaCl), Calcium chloride (CaCl2), etc.
Metallic Bond
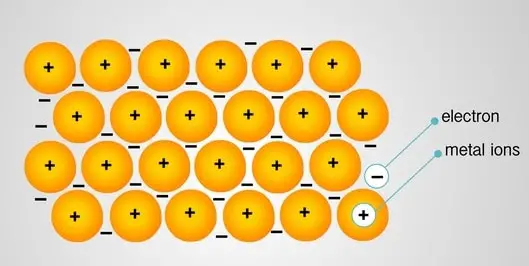
Metals have low ionization energy. Thus they release electrons easily as they are electropositive. These electrons leave their parent atoms and get delocalized. Metal bonding occurs among metal atoms.
For example, in a bar of sodium metal. Sodium has an electronic configuration of 1s2 2s2 2p6 3s1.
The 3s orbital is the outermost orbital and in the metal bar. When all the atoms are close together, the 3s orbital of one atom overlaps with the 3s orbital of the neighboring atom.
Thus the atomic orbitals overlap to form molecular orbitals. This overlap occurs with all the neighboring atoms on each side. Thus a huge number of molecular orbitals extend throughout the bar.
The electrons leave the 3s orbital and travel through the molecular orbitals, thus getting delocalized.
The metal is thus held together by the strong forces of attraction between the positive nuclei and delocalized electrons. Thus metallic bonding is very strong, and metals have high melting and boiling points.
Examples: Metallic bonding can also be found in all metals like copper, aluminum, etc.
Weak bonds
Hydrogen Bonding
A weak bond is formed when a hydrogen atom is directly bonded with a highly electronegative element like oxygen or fluorine.
In this case, the hydrogen acquires a partial positive charge as the electrons in the bond get more attracted to the electronegative atom.
If there is another electronegative atom nearby, the hydrogen atom gets attracted to the lone pairs of electrons on the nearby electronegative atom and forms a hydrogen bond.

The stronger the electronegativity difference, the stronger the bond.
Examples: This type of bonding can be found in Hydrofluoric acid (HF), water (H2O), etc.
Diploe-Dipole Interaction
In a covalent bond, if there is an electronegativity difference between the two atoms, then the electrons get pulled more towards the electronegative atom, and it has a higher electron density.
This results in a molecular dipole. The dipole moment is from the direction of the electropositive to the electronegative atom. When the dipole moments are not canceled due to the atom’s geometry, it becomes a polar atom.
In each molecule, one atom is partially positive, and the other is partially negative. The attractive forces between the partial positive of one atom and the partial negative of another atom are known as dipole-dipole interactions.
Example: These forces can be seen in Hydrochloric acids (HCl).
London dispersion forces
These are the weakest bonds formed due to intermolecular forces of attraction. These London dispersion forces are temporary forces of attraction formed between two adjacent atoms. These forces exist in both polar and non-polar molecules.