There are many types of gases in nature. Of them, some are natural, and others are manufactured. These include
- Hydrogen
- Oxygen
- Nitrogen
- Carbon dioxide
- Carbon monoxide
- Helium
- Neon
- Argon
- Krypton
- Methane
- Radon
- Fluorine
- Chlorine
- sulfur dioxide
- ammonia
- Nitrous oxide
- Acetylene
- Methyl chloride
- Dichlorodifluoromethane
- Hydrogen chloride, etc.
Definition of Gas
A gas is material with loosely bound molecules that flow from one place to another.
These gas molecules are widely separated from each other due to weak forces of attraction. Hence, they are invisible and lack a specific shape.
Since any form of volatile matter can be called a gas, the above-mentioned list is not complete.
Different Types of gases
There are basically two types as
- Natural gases (common gases)
- Artificial gases.
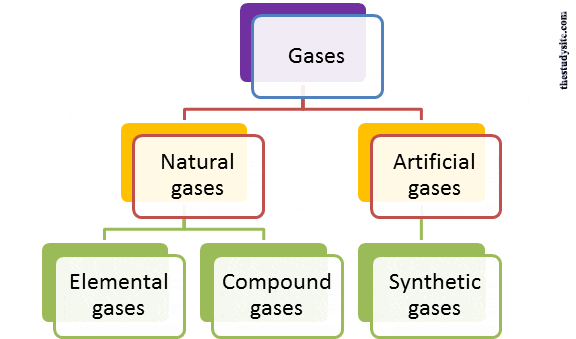
Natural gases
The gases include oxygen, nitrogen, hydrogen, etc., and are abundant in the air
Of these natural gases, some are elemental, while others are compounds.
Elemental gases
These gases are made of atoms of the same type
Examples are:
- Hydrogen (H2), [H+H]
- Oxygen (O2), [O+O]
- Nitrogen (N2), [N+N]
- Noble gases (Helium [He], Neon [Ne], Argon [Ar], krypton [Kr], and Radon [Rn].
Noble gases, unlike other gases, are monoatomic elements, which means they exist as individual atoms.
They are believed to be formed along with other matter on Earth.
In contrast, gases like Chlorine (Cl2) and fluorine (F2) exist in combination with other substances.
Compound gases
The molecules of these gases have different atoms. Yet they are present in nature and are formed through biochemical reactions.
They are made by a combination of either carbon, oxygen, nitrogen, hydrogen, or sulfur. Examples are
- Carbon dioxide-(CO2), [C+O+O]
- Methane-(CH4), [C+H+H+H+H]
- Sulfur dioxide-(SO2), [S+O+O]
- Ammonia-(NH3), [N+H+H+H], etc.
These gases are believed to have existed even before the start of life on Earth.
At the right concentration ratios, they are not harmful.
Natural gas
Fossil fuels are generally called natural gas. They are hydrocarbon mixtures comprising largely of methane and other higher alkanes.
They are extracted from the depths of earth and used as a source of energy for automobiles, cooking and electricity generation.
Synthetic gases
These gases are man made and synthesized through chemical reactions for industrial use.
These include
- Chlorofluorocarbons like R11, R12.
- Anesthetics like halothane, sevoflurane, desflurane, and isoflurane.
- Microbial Sterilizing agents like diethyl ether.
These are not essential to nature, and can even be harmful like causing ozone layer depletion.
Chlorofluorocarbons used in refrigerators harm the ozone layer, leading to its depletion.
This ozone layer protects us from the harmful UV rays of the sun rays from reaching the ground.
Further classification of gases
Cryogenic Gases
- These are the gases that can be used in liquid form. They have boiling point below -153 °C.
- These include nitrogen, oxygen, helium, hydrogen, neon etc.
- They are very cold and are used for different purposes like cold storage, metal hardening.
Acidic gases
- These gases are chemically acidic in nature. They have a pH below 7, and some of them are quite strong acids.
This includes
- Hydrochloric acid (HCl),
- Hydrofluoric acid (HF).
Since they are volatile, they are used as a mixture in water. They have a very low pH.
Basic gases
- Contrary to acidic gases, these have a pH of more than 7.
Ex: Ammonia (NH3).
Greenhouse gases
- These are gases that prevent the light radiation from the earth back into space and there by enhance the earth’s temperature.
- These gases include carbon dioxide, methane, nitrous oxide, etc.
- They lead to the greenhouse effect, wherein the temperature on the surface of the earth is high.
If the concentration of these gases increases, it can lead to global warming.
Uses of Gases
List of common gases and their uses.
| Name of Gas | Applications |
| Oxygen | Respiratory support, Welding, Sterilizer |
| Carbon Dioxide | Refrigerant, Anti-explosive in combination with other gases, |
| Nitrogen | Liquid nitrogen for cold storage, For oxygen-free environment, In respiratory support in health care. |
| Acetylene | Gas welding |
| Neon, Argon, Xenon | In electric bulbs and tubes |
| N2O (nitrous oxide) | Anaesthetic in dental surgery |
| Hydrogen | As fuel |
| Chlorine | As sterilizer |
| Natural Gas | Cooking |
| Sulfur dioxide | To preserve fruits, Winemaking |
In medicine
Gases are widely used in healthcare for respiration and anaesthesia.
To aid respiration
- In the case of respiratory insufficiency, oxygen therapy is given to sustain life. Respiratory insufficiency is a condition of the lungs with the inability to expand and contract.
- Due to this, they cannot take sufficient oxygen from the air spontaneously. This Respiratory insufficiency occurs in diseases like COPD and pneumonia.
- Here oxygen in combination with normal air, nitrogen, or even carbon dioxide is administered.
- Oxygen therapy helps old age people and also those with brain and spinal cord damage to recover from asphyxia.
Also, in tissue culture procedures, where tissue or cell is grown outside, the body gases are supplied. For this, especially oxygen, in combination with nitrogen, is suitable.
For anesthesia
- Gases like halothane, enflurane, ether, and chloroform are used to make the person lose consciousness.
- This loss of consciousness for the desired time is called anesthesia.
- It is especially important during operations, surgery, and even C-section delivery. The specialty is, they can be inhaled and the onset of anesthesia is very fast.
- Due to exhalation the recovery from anesthesia is fast too.
Since the induction and recovery are fast, these gaseous anaesthetics find regular use in medicine.
For chemical analysis
- Gases find their use even in the qualitative and quantitative analysis of compounds.
- Materials used in drug manufacture, nutrition, and research are analyzed. In gas chromatography, nitrogen is used as a carrier gas.
- While in flame photometry, gases like hydrogen, argon is used as they help in the ignition of the samples.
Other daily life activities
As fuel for cooking and other areas.
- For domestic cooking, natural gas is widely used. The gas produces a blue color flame of high temperature.
- Besides, this flame also does not form a black suit and smoke, unlike traditional fuels.
- Even for automobiles, there is research going on for the use of hydrogen as fuel.
- Similarly, Methane, a biomolecule obtained from biomass, is used to generate electricity.
For welding of metals.
Gases like oxygen, acetylene is used in metal welding, called gas welding.
For lights & lamps
- Most of the electric lamps we use have noble gases.
- The gases helium, xenon are commonly used in the lamp helps to prevent damage to the electrodes in the bulb due to high-velocity ions.
- These ions slow down the velocity of ions and thereby enhancing the lifespan of the filament in the tubes and bulbs.
For refrigerator (cooling)
- In refrigerators and air conditioners, gases like carbon dioxide, ammonia, chlorofluorocarbons, sulfur dioxide are used for cooling.
- These gases take the heat from within and release it out by condensing to liquid. They then convert to gas by taking the internal heat. Thus they convert from the liquid phase to gas phases alternatively.
- This way, they can aid in cooling the compartments in a fridge and also in air conditioners. They are used widely in storage, pharmaceutical industries, cars, and other automobiles.
For Cold transport
- Liquid gases are used to transfer thermosensitive substances.
- Liquid nitrogen has a temperature of -196° Celsius, while solid carbon dioxide has a temperature of -78.5° Celsius.
- This low temperature is suitable to keep the substances in a frozen state in a pack for a long time.
- Hence these are widely used in the transfer of foodstuff, biological tissue samples, microbes, etc.
For Sterilization
- Ethylene oxide is one of the gases used in microbial sterilization.
- These gases are toxic and kill all the possible microbes when exposed.
- Gaseous sterilization is especially useful for drug, food carrying containers.
- Once the drugs or food packets are arranged in containers for transport, these toxic gases are allowed to pass through.
- These gases move into every corner and gaps of the container and help ineffective sterilization.
In Research
- Gases are also used in research for experimental purposes.
- Nitrogen is used to create a non-oxygen atmosphere.
- Also, it is used to remove oxygen from solutions to study oxidation levels.
- In-plant tissue culture, to provide sterile oxygen supply to the growing plant tissue.
For purification and separation of substances
- When a solid or liquid is in impure state, it can be purified by converting it into another state.
- For example naphthalene is solid at room temperature and if it impure, it is converted to gas by the sublimation process.
- This gaseous form is re-cooled to solid in another container to obtain pure sample.
- Similarly, by distillation, we can obtain ion free water.
- If the material is heat-sensitive, it can be boiled at low temperature under vacuum to convert into gas, and then the gas is re-condensed to the original state of solid or liquid.
- Thus converting a substance into gas helps in its purification.
In agriculture
- Nitrogen is an important element used as fertilizer. It is converted from air into ammonia and fixed in the soil by some bacteria.
- This ammonia acts as a fertilizer to the crop. When the crop is destroyed, the gas is again returned to the atmosphere.
Similarly, in swimming pools and public water supplies, bleaching powder is used to kill disease-causing microbes.
- This bleaching powder, i.e., Ca (ClO)2, is a strong oxidizer. When mixed with water, it releases chlorine and oxygen gas atoms (O), that is highly toxic to microbes.
- This gas atoms kill the microbes and hence sanitize fresh water.
Also, room freshers, insect repellents, which we use regularly, emit volatile forms of gases.

Thank you for such a vital update. I was impressed
The artical was very helpfull for my online classes
Gases & Gasses-nice clarification!
It is very easy to understand all the forms of gases and its properties
Thanks
The article explains gases in an easy to understand manner. There are two plural forms of gas used throughout the article – gases and gasses. Each spelling has a specific meaning and gases would be appropriate throughout this article.
Gases is the plural of gas, which is a noun meaning a substance that expands to fill any container.
Gasses is a verb in the third-person present tense which carries a variety of meanings, e.g. She gasses up her car every Friday.
@Richard Grizzell ! Thanks for the correction.
Very useful knowledge…..
Thanks
differences between propane, natural, butane,gases and their dangers
Liquid gas from chemical like kerosen